X Chromosome Inactivation: A Breakthrough in Genetic Disorders
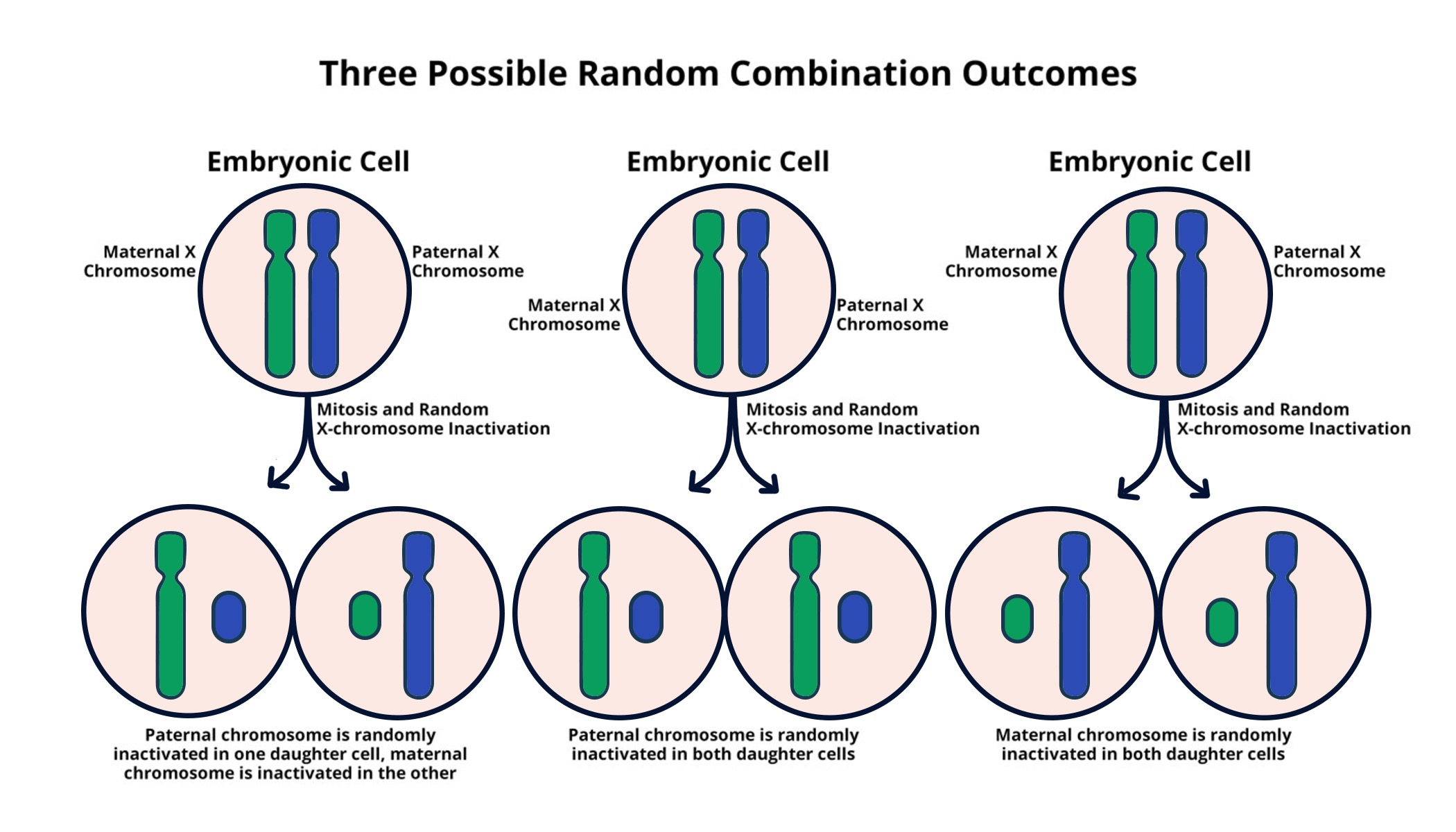
X chromosome inactivation is a fascinating biological process that plays a pivotal role in genetic regulation for females. Understanding this mechanism is crucial, especially in the context of genetic disorders like Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. Researchers like Jeannie T. Lee at Harvard Medical School have made groundbreaking strides in elucidating how cells effectively silence one of their two X chromosomes, ensuring that females do not express double the amount of X-linked genes in comparison to males. This chromosomal breakthrough opens the door to innovative treatments aimed at combating these challenging genetic disorders. By exploring the intricate dance of molecules involved in X chromosome inactivation, scientists are unlocking potential therapies that could transform the lives of many individuals affected by these conditions.
The process known as X chromosome silencing is essential in balancing gene expression in females, who possess two copies of this vital chromosome. This fascinating biological phenomenon addresses the unique challenges presented by the presence of an extra X in female cells and highlights the sophisticated methods cells employ to regulate gene function effectively. Notably, conditions like Fragile X and Rett syndromes underscore the critical implications of X chromosome inactivation, as these disorders arise from genetic mutations that reside on this chromosome. Pioneering researchers, including figures like Jeannie T. Lee, have contributed significantly to our understanding and management of such chromosomal complexities, leading to promising therapeutic avenues. As scientists continue to unravel the mysteries behind X-inactivation, the potential for novel treatments for genetic disorders remains firmly on the horizon.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a remarkable biological process that ensures dosage compensation in females. Since females possess two copies of the X chromosome while males have only one, this mechanism silences one X chromosome entirely in each cell to prevent an overexpression of X-linked genes. This process not only highlights the complexity of genetic regulation but also addresses how cells adapt to their genetic architecture. Jeannie T. Lee’s research has significantly contributed to this understanding, showcasing the pivotal role of RNA molecules such as Xist, which initiates the inactivation process by altering the physical properties of chromosomal structures.
The insights gained from XCI research have implications far beyond basic biology. As mutations can occur on X-linked genes leading to severe intellectual and neurodevelopmental disabilities—such as Fragile X Syndrome and Rett Syndrome—the potential for therapeutic breakthroughs becomes evident. Lee’s work illustrates how recognizing the mechanisms behind X chromosome inactivation can pave the way for developing targeted treatments that may activate dormant healthy genes locked away in the inactivated X chromosome.
The Role of Jeannie T. Lee in Chromosomal Research
Jeannie T. Lee, a prominent figure in genetic research, has dedicated her career to unraveling the complexities of X chromosome inactivation. As the vice chair of the Department of Genetics at Harvard Medical School, her pioneering work has opened new avenues in understanding genetic disorders linked to the X chromosome. The breakthroughs achieved in her laboratory are not only significant for academic knowledge but also hold promise for developing clinical applications to treat conditions like Fragile X Syndrome, where certain genetic markers directly affect patients’ health outcomes.
Lee’s research is backed by decades of investigation supported by the National Institutes of Health, focusing on fundamental genetic questions. This fruitful inquiry culminated in groundbreaking findings that elucidate how Xist RNA can modify the properties of chromatin—the Jell-O-like substance enveloping chromosomes. This understanding is crucial as it sheds light on cellular mechanisms and informs future strategies for gene therapy, particularly in the therapeutic context of genetic disorders resulting from faulty X-linked genes.
Implications of X Inactivation for Genetic Disorders
The implications of understanding X chromosome inactivation extend into the realm of genetic disorders, particularly those affecting intellectual capabilities and neurodevelopment. Fragile X Syndrome and Rett Syndrome are prime examples where the clinical significance of XCI can lead to promising treatment avenues. Researchers are now exploring how to un-silence the genes within the inactivated X chromosome to potentially restore function in affected individuals. This approach highlights the delicate balance between gene dosage and expression, which is quintessential in managing X-linked conditions.
Moreover, current methodologies developed in Lee’s lab aim to harness the mechanisms of XCI to create targeted interventions that can address specific mutations while sparing healthy gene functions. This idea not only revolutionizes how we think about gene therapy but also raises hopes for patients and families impacted by such genetic challenges. By manipulating the fabric of chromosomal interactions, there is potential not only for reversing symptoms but also for transforming the lives of those affected by these debilitating syndromes.
Mechanism of Chromosomal Silencing: A Scientific Perspective
The mechanism of chromosomal silencing introduced by Jeannie T. Lee’s research involves a sophisticated interplay of RNA molecules and the structural properties of chromatin. The gelatinous substance surrounding chromosomes, metaphorically compared to Jell-O, plays a crucial role in maintaining chromosomal integrity. Xist RNA engages with this material in a tug-of-war, resulting in the encapsulation and subsequent inactivation of the X chromosome. Dissecting this complex interaction reveals not just cellular biology but also the potential for therapeutic manipulation of these processes.
Understanding how chromosomal silencing is achieved lends insight into broader questions regarding gene expression regulation. This knowledge could inform strategies aiming to selectively activate genes stifled by mutations or faulty pathways. By elucidating these mechanisms, scientists stand on the brink of not only understanding genetic disorders better but also refining techniques to harness the body’s inherent systems for therapeutic influence.
Therapeutic Potential of Un-silencing Genes
The therapeutic potential of un-silencing genes through targeted manipulation of X chromosome inactivation is a groundbreaking leap forward in the field of medical genetics. For individuals with Fragile X Syndrome and Rett Syndrome, where specific mutations impede normal function, the research led by Lee offers hope for innovative treatment modalities. By unlocking the inactivated X chromosome, researchers aim to enable the expression of healthy genes that might otherwise remain dormant due to mutational interference.
Moreover, such therapies not only promise to ameliorate symptoms but also redefine the approaches taken towards treating genetic disorders at the molecular level. As ongoing research continues to explore the optimal methods for gene activation, the ultimate goal is to transition from laboratory insights to clinical applications, perhaps leading to personalized treatment plans that address the unique challenges faced by patients grappling with the effects of severe genetic mutations.
Navigating Mysteries of Genetic Expression
As researchers delve deeper into the nuances of genetic expression, the mysteries surrounding X chromosome inactivation present intriguing questions. One of the compelling aspects raised by Lee’s findings is how liberating inactivated genes restores function without disrupting healthy counterparts. This unique observation prompts further investigation into the cellular capacities for gene utilization, potentially unveiling new paradigms in genetic medicine.
The ongoing inquiry into these mechanisms signals a proactive approach to addressing genetic disorders. Understanding why certain genes remain unaffected during this ‘un-silencing’ could pave the way for more refined therapies that not only target specific gene functions but also consider the overall genetic landscape of individuals. The quest to navigate these intricacies exemplifies how continued research can lead to profound advancements in managing and treating genetic disorders.
Jell-O Like Substance and Chromosomal Functionality
The Jell-O-like substance that envelops chromosomes is a clever metaphor for the complex interactions present in genetic material. In Jeannie T. Lee’s research, this material is illustrated as flexible and adaptive, ideally suited to facilitate important biological functions such as gene silencing. For scientists, understanding how this substance behaves in relation to Xist RNA and other molecular players is pivotal for deciphering the mechanics of X chromosome inactivation.
Such insights have broader implications for numerous genetic conditions linked to the X chromosome, including Fragile X Syndrome. By examining the properties of this Jell-O-like substance, researchers can identify how modifications can enhance or hinder gene expression, contributing to the development of refined therapeutic strategies. Ultimately, the goal is to not only understand genetic mechanisms on a fundamental level but also to translate that knowledge into tangible treatments for patients.
Future Directions in Chromosomal Research
The landscape of chromosomal research is evolving rapidly, with Jeannie T. Lee’s contributions setting the stage for future investigational pathways. There is a growing sense of optimism that the mechanisms behind X chromosome inactivation will be further dissected, leading to invaluable insights into genetic disorders like Fragile X and Rett syndromes. The potential to manipulate these pathways opens the door to novel gene therapies that may reshape the treatment paradigm for various conditions.
As the research community gears up for clinical trials, the excitement surrounding these developments is palpable. Scientists are optimistic about optimizing the existing methods and ensuring safety as they move toward potential therapy applications. With collaborations expanding and funds dedicated to exploring these innovative strategies, the coming years could witness the realization of concepts that seem like science fiction but have strong foundations in emerging genetic research.
Conclusion: The Impact of Chromosomal Breakthroughs on Healthcare
The groundbreaking findings surrounding X chromosome inactivation and its implications for diseases like Fragile X Syndrome and Rett Syndrome mark a pivotal moment in genetic research and healthcare. The work of Jeannie T. Lee and her colleagues not only illuminates our understanding of basic biological mechanisms but also signifies a transition to practical applications in medicine. This intersection of basic research and clinical potential reflects a broader trend in medical sciences aiming at leveraging genetic insights to improve patient outcomes.
As we look towards the future, the excitement within the scientific community about these chromosomal breakthroughs underscores a commitment to tackling genetic disorders. Innovations in un-silencing genes present not just a chance for treatment but an invitation to redefine our approach to managing genetic health. Continuing this line of research promises to reshape our understanding and treatment of genetic disorders, ultimately fostering a more hopeful landscape for those affected.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to Fragile X Syndrome and Rett Syndrome?
X chromosome inactivation is a genetic process that occurs in females, where one of the two X chromosomes is randomly silenced to prevent the overexpression of X-linked genes. This mechanism is particularly relevant in the context of disorders like Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. Understanding how X chromosome inactivation works can help develop therapies for these genetic disorders.
How does Jeannie T. Lee’s research contribute to our understanding of X chromosome inactivation?
Jeannie T. Lee’s research at Harvard Medical School has significantly advanced our understanding of X chromosome inactivation. Her lab discovered that a gelatinous substance coats chromosomes, allowing for the silencing of one X chromosome in females. Insights from her studies could lead to potential treatments for genetic disorders like Fragile X Syndrome and Rett Syndrome by potentially un-silencing mutated X-linked genes.
Can X chromosome inactivation be reversed to treat genetic disorders?
Recent findings suggest that liberating inactivated X chromosomes may restore the function of mutated genes without adversely affecting healthy genes. This breakthrough in understanding X chromosome inactivation could lead to potential treatments for genetic disorders such as Fragile X Syndrome and Rett Syndrome, as it enables access to the functional versions of the genes that are otherwise silenced.
What implications does X chromosome inactivation have for males with X-linked genetic disorders?
While males typically have only one X chromosome and do not undergo X chromosome inactivation, they can still be affected by mutations on their single X chromosome. Understanding X chromosome inactivation opens avenues for therapeutic strategies that could target individual genes on the X chromosome, potentially benefiting males with X-linked genetic disorders like Fragile X Syndrome.
Why is the jelly-like substance important in the process of X chromosome inactivation?
The jelly-like substance surrounding chromosomes plays a crucial role in X chromosome inactivation by preventing chromosomes from tangling and allowing molecules like Xist to modify its properties. This alteration enables the efficient silencing of one X chromosome. Jeannie T. Lee’s lab demonstrated that the flexibility of this substance is essential for the biochemical processes required to make the X chromosome inactive.
What are the potential therapeutic applications of manipulating X chromosome inactivation?
Manipulating X chromosome inactivation has significant therapeutic potential for treating X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome. By targeting the processes involved in X inactivation, researchers aim to un-silence the healthy versions of genes that are currently inaccessible due to inactivation, paving the way for innovative treatments that could improve patient outcomes.
How long has research on X chromosome inactivation been ongoing, and what recent breakthroughs have been made?
Research on X chromosome inactivation has been ongoing for over 25 years, with foundational studies conducted by Jeannie T. Lee and her team. Recent breakthroughs include the understanding of the role of the gelatinous substance surrounding chromosomes and the possibility of un-silencing mutated genes on the X chromosome, leading to prospective therapeutic applications for genetic disorders.
What challenges remain in the study of X chromosome inactivation and its implications for genetic disorders?
Despite advancements in understanding X chromosome inactivation, challenges remain in elucidating why un-silencing processes do not affect all genes uniformly. Further research is needed to determine the mechanisms that allow certain genes to remain functional while others are silenced. Addressing these challenges could enhance the development of targeted treatments for disorders linked to mutations on the X chromosome.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes but only need one, so one is inactivated. |
| Role of Gelatinous Substance | A substance described as ‘Jell-O’ separates chromosomes, preventing them from tangling. |
| Xist RNA Role | Xist alters the Jell-O’s properties, allowing inactivation of the X chromosome. |
| Potential Treatments | Research may lead to treatments for Fragile X and Rett syndromes by liberating inactivated genes. |
| Clinical Trials | Optimizing methods for un-silencing genes for clinical research. |
| Mysteries of Gene Usage | Uncertain why inactivated X chromosomes can restore function with minimal side effects. |
Summary
X chromosome inactivation is a crucial process in mammalian genetics that ensures females with two X chromosomes do not produce double the amount of gene products compared to males. This complex mechanism, which Jeannie T. Lee’s research has illuminated, relies on a gelatinous substance that modifies the structural behavior of the X chromosome. This not only advances our understanding of gene regulation but also opens avenues for potential therapies targeting disorders linked to the X chromosome, such as Fragile X and Rett syndromes. With ongoing research, we may be able to safely reactivate crucial genes, hence providing more effective treatment options for those affected.